cis-nerolidol
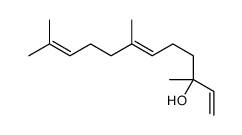
cis-nerolidol structure
|
Common Name | cis-nerolidol | ||
|---|---|---|---|---|
| CAS Number | 3790-78-1 | Molecular Weight | 222.37 | |
| Density | 0.869g/cm3 | Boiling Point | 276ºC at 760mmHg | |
| Molecular Formula | C15H26O | Melting Point | -98.0 °C (lit.) | |
| MSDS | USA | Flash Point | 109.9ºC | |
Use of cis-nerolidolcis-Nerolidol is a sesquiterpene alcohol that can be found in various plants. cis-Nerolidol exhibits antioxidant and antibacterial activities. cis-Nerolidol can also potentiate the action of antibiotics[1]. |
| Name | (6Z)-3,7,11-trimethyldodeca-1,6,10-trien-3-ol |
|---|---|
| Synonym | More Synonyms |
| Description | cis-Nerolidol is a sesquiterpene alcohol that can be found in various plants. cis-Nerolidol exhibits antioxidant and antibacterial activities. cis-Nerolidol can also potentiate the action of antibiotics[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 0.869g/cm3 |
|---|---|
| Boiling Point | 276ºC at 760mmHg |
| Melting Point | -98.0 °C (lit.) |
| Molecular Formula | C15H26O |
| Molecular Weight | 222.37 |
| Flash Point | 109.9ºC |
| Exact Mass | 222.19800 |
| PSA | 20.23000 |
| LogP | 4.39630 |
| Index of Refraction | n20/D 1.478(lit.) |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2905290000 |
| HS Code | 2905290000 |
|---|---|
| Summary | 2905290000 unsaturated monohydric alcohols。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:5.5%。General tariff:30.0% |
|
Capturing of the monoterpene olefin limonene produced in Saccharomyces cerevisiae.
Yeast , doi:10.1002/yea.3038, (2014) Monoterpene olefins such as limonene are plant compounds with applications as flavouring and fragrance agents, as solvents and potentially also in polymer and fuel chemistry. We engineered baker's yea... |
|
|
[Analyze on chemical compositions of Dalbergia odorifera essential oils extracted by CO2-supercritical-fluid-extraction and steam distillation extraction].
Zhong Yao Cai 34(11) , 1725-7, (2011) To analyze the chemical compositions of Dalbergia odorifera essential oils extacted by CO2-supercritical-fluid-extraction (SFE-CO2) and steam distillation extraction (SD).The essential oils of Dalberg... |
|
|
Transdermal behaviors comparisons among Evodia rutaecarpa extracts with different purity of evodiamine and rutaecarpine and the effect of topical formulation in vivo.
Fitoterapia 83(5) , 954-60, (2012) Evodiamine (EVO) and rutaecapine (RUT), the major active components from Evodia rutaecarpa extract (EE), are recognized as a depended analgesic agent. This study was designed to investigate the effect... |
| (+-)-3,7,11-trimethyl-dodeca-1,6c,10-trien-3-ol |
| 1,6,10-Dodecatrien-3-ol,3,7,11-trimethyl-,[S-(Z)] |
| (Z)-nerolidol |
| Nerolidol cis-form |
| cis-Nerolidol |
| 1,6,10-Dodecatrien-3-ol,3,7,11-trimethyl-,(Z)-(S)-(+) |
| Nerolidol isomer |
| (Z)-3,7,11-trimethyl-dodeca-1,6,10-trien-3-ol |
| cis-3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol |
| (3RS,Z)-Nerolidol |
 CAS#:515-69-5
CAS#:515-69-5 CAS#:80767-67-5
CAS#:80767-67-5 CAS#:100664-26-4
CAS#:100664-26-4 CAS#:1117-52-8
CAS#:1117-52-8 CAS#:626-96-0
CAS#:626-96-0 CAS#:67-64-1
CAS#:67-64-1
