C12 NBD-SPHINGOMYELIN
Modify Date: 2024-01-23 13:55:31
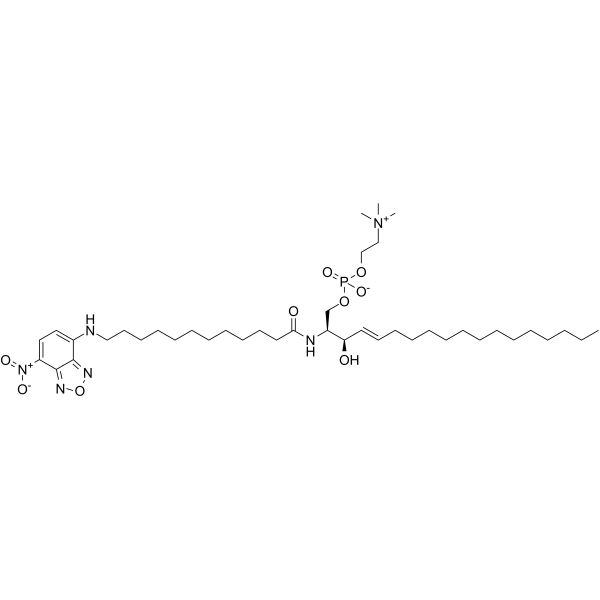
C12 NBD-SPHINGOMYELIN structure
|
Common Name | C12 NBD-SPHINGOMYELIN | ||
|---|---|---|---|---|
| CAS Number | 254117-01-6 | Molecular Weight | 825.027 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C41H73N6O9P | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of C12 NBD-SPHINGOMYELINC12 NBD sphingomyelin is an active derivative of sphingomyelin (HY-113498) that is tagged with fluorescent C12 nitrobenzoxadiazole (C12 NBD). C12 NBD sphingomyelin can be used as a sphingomyelinase substrate for studying the metabolism and transport of sphingomyelins (Ex=470 nm, Em=525 nm)[1]. |
| Name | (2S,3R,4E)-3-Hydroxy-2-({12-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]dodecanoyl}amino)-4-octadecen-1-yl 2-(trimethylammonio)ethyl phosphate |
|---|---|
| Synonym | More Synonyms |
| Description | C12 NBD sphingomyelin is an active derivative of sphingomyelin (HY-113498) that is tagged with fluorescent C12 nitrobenzoxadiazole (C12 NBD). C12 NBD sphingomyelin can be used as a sphingomyelinase substrate for studying the metabolism and transport of sphingomyelins (Ex=470 nm, Em=525 nm)[1]. |
|---|---|
| Related Catalog | |
| Target |
Sphingomyelinase[1] |
| In Vitro | Guidelines (Following is our recommended protocol. This protocol only provides a guideline, and should be modified according to your specific needs)[1]. Assay for Sphingolipid-Degrading Enzymes (EGCase Ⅱ, SCDase and SMase): 1. Incubate amounts of enzymes with 0.1 nM dye at 37 ℃ for indicated times under following conditions. (1). 10 mM sodium acetate buffer (pH5.0) containing 0.2% Triton X-100 for EGCase. (2) 25 mM sodium phosphate buffer (pH 6.0) containing 0.1% Triton X-100 for SCDase. (3) 25 mM sodium phosphate buffer (pH 7.0) containing 0.2% Triton X-100 for SMase. 2. After incubation, the solvent is evaporated and the residue is dried, dissolved in 10 μL of chloroform/methanol (2:1) and analyzed by TLC using chloroform/methanol/0.02% CaCl2 (5:4:1, v/v) as the developing solvent. 3. Degradation products and remaining substrates are separated by TLC and quantified with a chromatoscanner (excitation 470 nm, emission 525 nm) for fluorescence-labeled substrates. |
| References |
| Molecular Formula | C41H73N6O9P |
|---|---|
| Molecular Weight | 825.027 |
| Exact Mass | 824.517639 |
| LogP | 7.63 |
| (2S,3R,4E)-3-Hydroxy-2-({12-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]dodecanoyl}amino)-4-octadecen-1-yl 2-(trimethylammonio)ethyl phosphate |
| Ethanaminium, 2-[[hydroxy[[(2S,3R,4E)-3-hydroxy-2-[[12-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]-1-oxododecyl]amino]-4-octadecen-1-yl]oxy]phosphinyl]oxy]-N,N,N-trimethyl-, inner salt |