| Description |
Lapatinib (GW572016) is a potent EGFR and ErbB2 inhibitor with IC50s of 10.2 and 9.8 nM, respectively.
|
| Related Catalog |
|
| Target |
EGFR:10.2 nM (IC50, Cell Free Assay)
ErbB2:9.8 nM (IC50, Cell Free Assay)
|
| In Vitro |
The IC50 of Lapatinib (GW2016) values for inhibition of enzyme activity are generated by measuring inhibition of phosphorylation of a peptide substrate. With the exception of ErbB-4 (IC50, 367 nM), Lapatinib is >300-fold selective for EGFR and ErbB-2 over other kinases tested[1]. IC50 values of Lapatinib (GW2016) for BT474, SKBR3, EFM192A, HCC1954, MDAMB453 and MDAMB231 cells is 36±15.1 nM, 80±17.3 nM, 193±66.5 nM, 416.6±180 nM, 6.08±0.825 μM and 7.46±0.102 μM, respectively. Treatment with Lapatinib results in IC50 values of ≤ 0.16 μM on the EGFR- and the ErbB-2-overexpressing tumor cell lines[2].
|
| In Vivo |
Lapatinib (GW2016) is potent at inhibiting the growth of BT474 and HN5 human tumor xenografts. A dose-responsive inhibition of both models occurred on treatment of tumor-bearing mice with 30 and 100 mg/kg Lapatinib orally, twice daily. Complete inhibition of tumor growth is seen at the 100 mg/kg dose. At this dose, there is <10% weight loss in treated animals over the course of the 21-day treatment. Lapatinib treatment inhibits tumor xenograft growth of the HN5 and BT474 cells in a dose-responsive manner at 30 and 100 mg/kg orally, twice daily, with complete inhibition of tumor growth at the higher dose[1]. Lapatinib (100 mg/kg/day, oral gavage) induces severe oxidative damage in the cardiac tissue of rat[3].
|
| Kinase Assay |
The intracellular kinase domains of EGFR, ErbB-2, and ErbB4 are purified from a baculovirus expression system. EGFR, ErbB-2, and ErbB-4 reactions are performed in 96-well polystyrene round-bottomed plates in a final volume of 45 μL. Reaction mixtures contain 50 mM 4-morpholinepropanesulfonic acid (pH 7.5), 2 mM MnCl2, 10 μM ATP, 1 μCi of [γ-33P] ATP/reaction, 50 μM Peptide A [Biotin-(amino hexonoic acid)-EEEEYFELVAKKK-CONH2], 1 mM dithiothreitol, and 1 μL of DMSO containing serial dilutions of Lapatinib beginning at 10 μM. The reaction is initiated by adding the indicated purified type-1 receptor intracellular domain. The amount of enzyme added is 1 pmol/reaction (20 nM). Reactions are terminated after 10 min at 23°C by adding 45 μL of 0.5% phosphoric acid in water. The terminated reaction mix (75 μL) is transferred to phosphocellulose filter plates. The plates are filtered and washed three times with 200 μL of 0.5% phosphoric acid. Scintillation cocktail (50 μL) is added to each well, and the assay is quantified by counting in a Packard Topcount[1].
|
| Cell Assay |
Cells are plated in 96-well plates, in the media, at the following densities: HFF and HN5, 1000 cells/well and BT474, 5000 cells/well. After 24 h, the cells are exposed to vehicle (0.3% DMSO) or Lapatinib (1 nM, 10 nM, 100 nM, 1μM, 10μM, and 100μM). Lapatinib is removed from the cells after 72 h and is replaced by either DMEM containing 10% FBS and 50 μg/mL Gentamicin (HFF and HN5) or RPMI containing 10% FBS and 50 μg/mL Gentamicin (BT474). Methylene blue staining is performed at the time points over a total period of 16 days[1].
|
| Animal Admin |
Mice[1] CD-1 nude female mice are used for HN5 human tumor xenografts, which are initiated by injection of a cell suspension in PBS:Matrigel (1:1). C.B-17 SCID female mice are used for BT474 human tumor xenografts, which are initiated by implantation of tumor fragments (20-100 mg) from established tumors. Tumor cells and fragments are implanted by s.c. injection in the right flank. The s.c. tumors are measured with calipers, and mice are weighed twice weekly. Tumor weight is estimated from tumor volume using this formula: length×width2/2=tumor volume (mm3). Treatment begins when tumors are palpable, 3-5 mm in diameter. Lapatinib (30 and 100 mg/kg) is administered p.o. twice daily for 21 days in a vehicle of sulfo-butyl-ether-β-cyclodextrin 10% aqueous solution (CD10). Rats[3] Wistar rats (12-week-old albino males) are randomly assigned to three groups: control (C, n=8), Trastuzumab (T, n=8) and Lapatinib (L, n=8) treatments. The control animals are untreated, but the others in groups T and Lapatinib are administered with the chemotherapy drugs. Trastuzumab is delivered once at a dose of 10 mg/kg/day via intraperitoneal injection on the first day of the study. Lapatinib is administered daily at a dose of 100 mg/kg/day by oral gavage for 7 consecutive days. The selected doses are equivalent to those used in the clinics. On day 8, anesthesia is induced by a single intraperitoneal injection of ketamine and xylazine (50 and 5 mg/kg, respectively). The blood samples are collected and the hearts are removed for biochemical analysis.
|
| References |
[1]. Rusnak DW, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001 Dec;1(2):85-94 [2]. O'Neill F, et al. Gene expression changes as markers of early lapatinib response in a panel of breast cancer cell lines. Mol Cancer. 2012 Jun 18;11(1):41. [3]. Eryilmaz U, et al. S100A1 as a Potential Diagnostic Biomarker for Assessing Cardiotoxicity and Implications for the Chemotherapy of Certain Cancers. PLoS One. 2015 Dec 18;10(12):e0145418. [4]. Ni J, et al. Combination inhibition of PI3K and mTORC1 yields durable remissions in mice bearing orthotopic patient-derived xenografts of HER2-positive breast cancer brain metastases. Nat Med. 2016 Jul;22(7):723-6. [5]. Oncogene. 2016 Jun 9;35(23):2961-70. doi: 10.1038/onc.2015.377. Epub 2015 Dec 7.
|
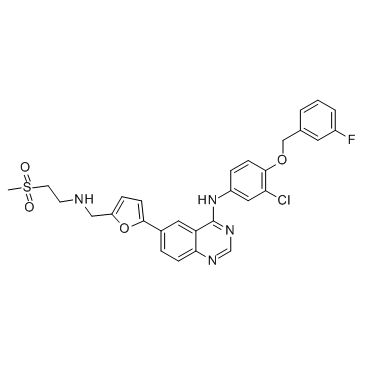
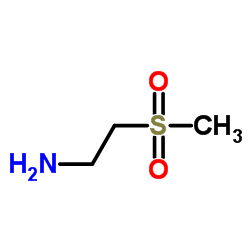 CAS#:49773-20-8
CAS#:49773-20-8![5-[4-[3-chloro-4-[(3-fluorophenyl)methoxy]anilino]quinazolin-6-yl]furan-2-carbaldehyde Structure](https://www.chemsrc.com/caspic/482/231278-84-5.png) CAS#:231278-84-5
CAS#:231278-84-5 CAS#:388082-78-8
CAS#:388082-78-8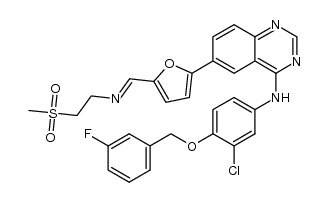 CAS#:1227853-06-6
CAS#:1227853-06-6 CAS#:104458-24-4
CAS#:104458-24-4 CAS#:388082-76-6
CAS#:388082-76-6 CAS#:1334953-75-1
CAS#:1334953-75-1![3-Chloro-4-[(3-fluorobenzyl)oxy]aniline Structure](https://www.chemsrc.com/caspic/081/202197-26-0.png) CAS#:202197-26-0
CAS#:202197-26-0 CAS#:231278-20-9
CAS#:231278-20-9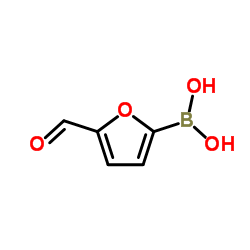 CAS#:27329-70-0
CAS#:27329-70-0
