CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YT4500000
-
CHEMICAL NAME :
-
Urea, 1-(hexahydrocyclopenta(c)pyrrol-2(1H)-yl)-3-(p-tolyls ulfonyl)-
-
CAS REGISTRY NUMBER :
-
21187-98-4
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
15
-
MOLECULAR FORMULA :
-
C15-H21-N3-O3-S
-
MOLECULAR WEIGHT :
-
323.45
-
WISWESSER LINE NOTATION :
-
T55 CNTJ CMVMSWR D1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
9600 ug/kg/24D-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - acute pulmonary edema Kidney, Ureter, Bladder - urine volume decreased Nutritional and Gross Metabolic - other changes
-
REFERENCE :
-
IEDIEP Internal Medicine. (The Japanese Society of Internal Medicine, 34-3, 3-chome, Hongo, Bunkyo-ku, Tokyo 113, Japan) V.31- 1992- Volume(issue)/page/year: 33,163,1994
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
INDRBA Indian Drugs. (Indian Drugs Manufacturers' Assoc., 102B, Poonam Chambers, Dr. A. B. Rd., Worli, Bombay 400 018, India) V.1- 1963- Volume(issue)/page/year: 15,161,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 8,2661,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
382 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 8,2661,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
INDRBA Indian Drugs. (Indian Drugs Manufacturers' Assoc., 102B, Poonam Chambers, Dr. A. B. Rd., Worli, Bombay 400 018, India) V.1- 1963- Volume(issue)/page/year: 15,161,1978
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>1 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
EJMCA5 European Journal of Medicinal Chemistry--Chimie Therapeutique. (Editions Scientifiques Elsevier, 29 rue Buffon, F-75005, Paris, France) V.9- 1974- Volume(issue)/page/year: 17,81,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1034 mg/kg
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Eye) - lacrimation Behavioral - somnolence (general depressed activity)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 8,2661,1980
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
295 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 8,2661,1980 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
48 gm/kg/30D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume increased Liver - changes in liver weight Blood - normocytic anemia
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 8,2661,1980
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
36 gm/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume increased Nutritional and Gross Metabolic - weight loss or decreased weight gain Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - phosphatases
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 8,2661,1980 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
4400 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system Reproductive - Effects on Newborn - behavioral
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,3551,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
2200 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,3551,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1100 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,3551,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 17-22 day(s) after conception lactating female 4 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - viability index (e.g., # alive at day 4 per # born alive)
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,3551,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1560 mg/kg
-
SEX/DURATION :
-
female 6-18 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 9,3551,1981
|
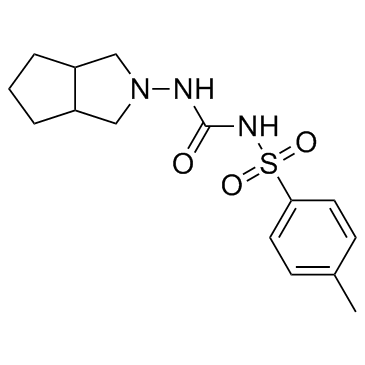
 CAS#:70-55-3
CAS#:70-55-3![3-Amino-3-azabicyclo[3.3.0]octane hydrochloride structure](https://www.chemsrc.com/caspic/478/58108-05-7.png) CAS#:58108-05-7
CAS#:58108-05-7
