2,4,4,6-tetrabromo-2,5-cyclohexadienone
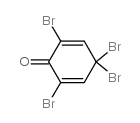
2,4,4,6-tetrabromo-2,5-cyclohexadienone structure
|
Common Name | 2,4,4,6-tetrabromo-2,5-cyclohexadienone | ||
|---|---|---|---|---|
| CAS Number | 20244-61-5 | Molecular Weight | 409.69500 | |
| Density | 2.897 g/cm3 | Boiling Point | 365.8ºC at 760 mmHg | |
| Molecular Formula | C6H2Br4O | Melting Point | 120-127 °C | |
| MSDS | Chinese USA | Flash Point | 136ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 2,4,4,6-tetrabromocyclohexa-2,5-dien-1-one |
|---|---|
| Synonym | More Synonyms |
| Density | 2.897 g/cm3 |
|---|---|
| Boiling Point | 365.8ºC at 760 mmHg |
| Melting Point | 120-127 °C |
| Molecular Formula | C6H2Br4O |
| Molecular Weight | 409.69500 |
| Flash Point | 136ºC |
| Exact Mass | 405.68400 |
| PSA | 17.07000 |
| LogP | 3.61280 |
| Vapour Pressure | 1.53E-05mmHg at 25°C |
| Index of Refraction | 1.757 |
| Storage condition | 0-6°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2914700090 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
A new approach to the reduction of sulfoxides to sulfides with 1,3-dithiane in the presence of electrophilic bromine as catalyst.
J. Org. Chem. 67(9) , 2826-30, (2002) A new, mild, and novel method is described for the efficient deoxygenation of sulfoxides to their corresponding sulfides with 1,3-dithiane at room temperature in the presence of catalytic amounts of N... |
|
|
Spectrophotometric and thermal studies on the charge--transfer complexes of 4-(aminomethyl) piperidine as donor with σ- and π-electron acceptors.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 118 , 1012-9, (2014) The spectroscopic characteristics of the solid charge-transfer molecular complexes (CT) formed in the reaction of the electron donor 4-(aminomethyl) piperidine (4AMP) with the σ-acceptor iodine and th... |
|
|
An efficient method for converting alcohols to azides with 2, 4, 4, 6-tetrabromo-2, 5-cyclohexadienone/PPh3 /Zn (N3)2· 2Py. Saito A, et al.
Tetrahedron Lett. 38(22) , 3955-58, (1997)
|
| 2,4,4,6-tetrabromo-2,5-cyclohexadieneone |
| 2,4,4,6-Tetrabromocyclohexa-2,5-dienone |
| EINECS 243-638-7 |
| 2,4,4,6-Tetrabromo-2,5-cyclohexadien-1-one |
| MFCD00001589 |
| 2,4,4,6-TetrabroMo-2,5-cyclohexadienone |
| 2,3-DIFLUOROPHENYLGLYCINE |
 CAS#:118-79-6
CAS#:118-79-6 CAS#:108-95-2
CAS#:108-95-2 CAS#:106-41-2
CAS#:106-41-2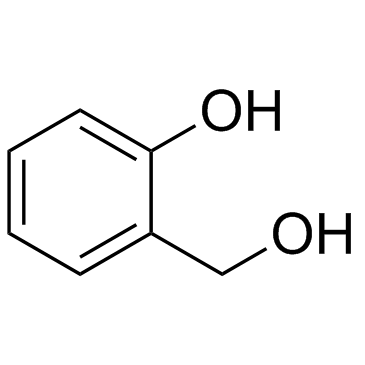 CAS#:90-01-7
CAS#:90-01-7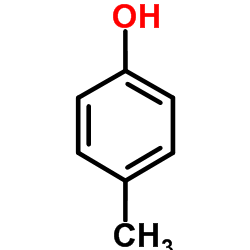 CAS#:106-44-5
CAS#:106-44-5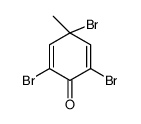 CAS#:39953-10-1
CAS#:39953-10-1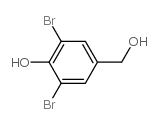 CAS#:2316-62-3
CAS#:2316-62-3 CAS#:5532-75-2
CAS#:5532-75-2 CAS#:2432-14-6
CAS#:2432-14-6 CAS#:69-72-7
CAS#:69-72-7 CAS#:367-24-8
CAS#:367-24-8 CAS#:607-99-8
CAS#:607-99-8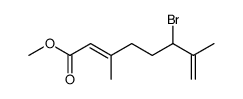 CAS#:75107-35-6
CAS#:75107-35-6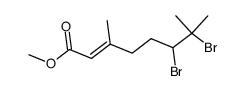 CAS#:77889-80-6
CAS#:77889-80-6 CAS#:59557-91-4
CAS#:59557-91-4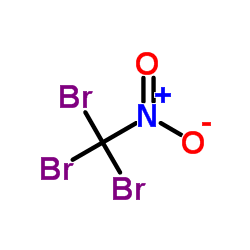 CAS#:464-10-8
CAS#:464-10-8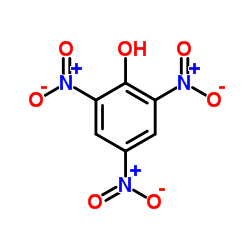 CAS#:88-89-1
CAS#:88-89-1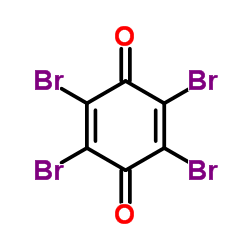 CAS#:488-48-2
CAS#:488-48-2