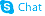Irinotecan hydrochloride
Irinotecan hydrochloride structure
|
Common Name | Irinotecan hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 100286-90-6 | Molecular Weight | 623.139 | |
| Density | N/A | Boiling Point | 257 °C | |
| Molecular Formula | C33H39ClN4O6 | Melting Point | 250-256°C (dec.) | |
| MSDS | Chinese USA | Flash Point | 482ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Irinotecan hydrochlorideIrinotecan hydrochloride is a water soluble topoisomerase I inhibitor mainly used to treat colon cancer and rectal cancer. |
| Name | Irinotecan hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Irinotecan hydrochloride is a water soluble topoisomerase I inhibitor mainly used to treat colon cancer and rectal cancer. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase I |
| In Vitro | Irinotecan hydrochloride is a topoisomerase I inhibitor. Irinotecan inhibits the growth of LoVo and HT-29 cells, with IC50s of 15.8 ± 5.1 and 5.17 ± 1.4 μM, respectively, and induces similar amounts of cleavable complexes in both in LoVo and HT-29 cells[2]. Irinotecan suppresses the proliferation of human umbilical vein endothelial cells (HUVEC), with an IC50 of 1.3 μM[3]. |
| In Vivo | Irinotecan (CPT-11, 5 mg/kg) significantly inhibits the growth of tumors by intratumoral injection daily for 5 days, on two consecutive weeks in rats, and such effects also occur via continuous intraperitoneal infusion by osmotic minipump into mice. However, Irinotecan (10 mg/kg) shows no effect on the growth of tumor by i.p[1]. Irinotecan (CPT-11, 100-300 mg/kg, i.p.) apparently suppresses tumor growth of HT-29 xenografts in athymic female mice by day 21. The two groups of Irinotecan (125 mg/kg) plus TSP-1 (10 mg/kg per day) or Irinotecan (150 mg/kg) in combination TSP-1 (20 mg/kg per day) are nearly equally effective and inhibit tumor growth 84% and 89%, respectively, and both are more effective than Irinotecan alone at doses of 250 and 300 mg/kg[3]. |
| Cell Assay | Exponentially growing cells are seeded in 20 cm2 dishes with an optimal cell number for each cell line (20,000 for LoVo cells, 100,000 for HT-29 cells). They are treated 2 days later with increasing concentrations of irinotecan or SN-38 for one cell doubling time (24 h for LoVo cells, 40 h for HT-29 cells). After washing with 0.15 M NaCl, the cells are further grown for two doubling times in normal medium, detached from the support with trypsin-EDTA and counted in a hemocytometer. The IC50 values are then estimated as the drug concentrations responsible for 50% growth inhibition as compared with cells incubated without drug[2]. |
| Animal Admin | Irinotecan has been administered by intratumoral injection at 0.1 cc volume of the appropriate solution, for a doses of 5 mg/kg daily for 5 days, on two consecutive weeks, followed by a 7-days rest period, referred to as one cycle of therapy. Rats receive three cycles over a period of 8 weeks. Control animals receive 0.1 cc of sterile 0.9% sodium chloride solution by intratumoral injection in the same rule of administration as that of animals of group II[1]. |
| References |
| Boiling Point | 257 °C |
|---|---|
| Melting Point | 250-256°C (dec.) |
| Molecular Formula | C33H39ClN4O6 |
| Molecular Weight | 623.139 |
| Flash Point | 482ºC |
| Exact Mass | 622.255798 |
| PSA | 114.20000 |
| LogP | 4.76890 |
| Vapour Pressure | 1.31E-32mmHg at 25°C |
| Index of Refraction | 67.7 ° (C=1, H2O) |
| Storage condition | Refrigerator |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Precursor 10 | |
|---|---|
| DownStream 2 | |
|
Aqueous-core PEG-coated PLA nanocapsules for an efficient entrapment of water soluble anticancer drugs and a smart therapeutic response.
Eur. J. Pharm. Biopharm. 89 , 30-9, (2015) Novel PEGylated PLA nanocapsules (PEG-AcPLA nanocapsules), loading high percentage of water soluble drugs have been formulated by using multiple emulsion technique without using conventional stabilize... |
|
|
Assessment of the efficacy of anticancer drugs by amino acid metabolomics using fluorescence derivatization-HPLC.
Anal. Sci. 30(7) , 751-8, (2014) Metabolomic studies conducted for evaluating cancer pathogenesis and progression by monitoring the amino acids metabolic balance hold great promise for assessing current and future anticancer treatmen... |
|
|
Evaluation of the in vitro/in vivo potential of five berries (bilberry, blueberry, cranberry, elderberry, and raspberry ketones) commonly used as herbal supplements to inhibit uridine diphospho-glucuronosyltransferase.
Food Chem. Toxicol. 72 , 13-9, (2014) In this study, we evaluated inhibitory potentials of popularly-consumed berries (bilberry, blueberry, cranberry, elderberry, and raspberry ketones) as herbal supplements on UGT1A1, UGT1A4, UGT1A6, UGT... |
| Campto hydrochloride |
| Campto |
| [1,4'-Bipiperidine]-1'-carboxylic acid, (4S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl ester, hydrochloride (1:1) |
| [1,4'-bipiperidine]-1'-carboxylic acid, (4S)-4,11-diethyl-3,4,12,14-tetrahydro-4-hydroxy-3,14-dioxo-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl ester, monohydrochloride |
| 1,4'-bipipéridine-1'-carboxylate de (4S)-4,11-diéthyl-4-hydroxy-3,14-dioxo-3,4,12,14-tétrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoléin-9-yle chlorhydrate |
| Camptothecin 11 hydrochloride |
| Irinotecan hydrochloride |
| CPT-11 hydrochloride |
| Topotecin |
| (4S)-4,11-Diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl 1,4'-bipiperidine-1'-carboxylate hydrochloride |
| irinotecan hydrochloride (anhydrous) |
| Irinotecan Hcl |
| CPT 11 |
| (4s)-4,11-diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1h-pyrano[3',4':6,7]indolizino[1,2-b]chinolin-9-yl-1,4'-bipiperidin-1'-carboxylathydrochlorid |
| (4S)-4,11-Diethyl-4-hydroxy-3,14-dioxo-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinolin-9-yl 1,4'-bipiperidine-1'-carboxylate hydrochloride (1:1) |
| Camptothecin analog |
| MFCD01862255 |
| Irinotecan (hydrochloride) |
 CAS#:4897-50-1
CAS#:4897-50-1 CAS#:86639-52-3
CAS#:86639-52-3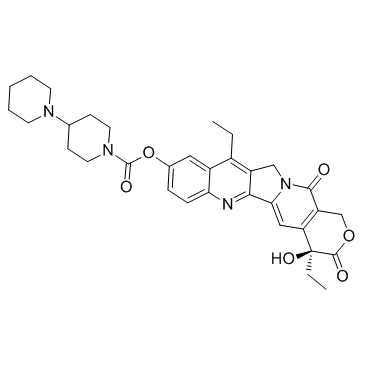 CAS#:97682-44-5
CAS#:97682-44-5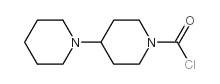 CAS#:103816-19-9
CAS#:103816-19-9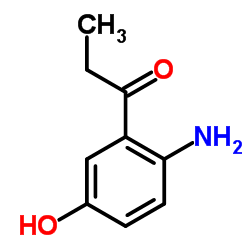 CAS#:35364-15-9
CAS#:35364-15-9![(S)-4-ethyl-4-hydroxy-7,8-dihydro-1H-pyrano[3,4-f]indolizine-3,6,10(4H)-trione Structure](https://www.chemsrc.com/caspic/225/110351-94-5.png) CAS#:110351-94-5
CAS#:110351-94-5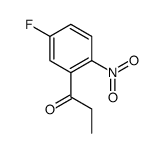 CAS#:924648-17-9
CAS#:924648-17-9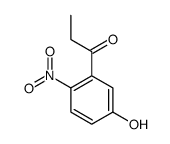 CAS#:453518-19-9
CAS#:453518-19-9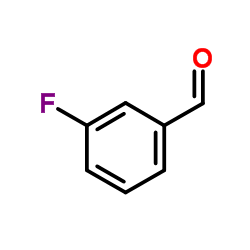 CAS#:456-48-4
CAS#:456-48-4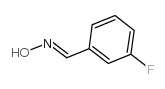 CAS#:458-02-6
CAS#:458-02-6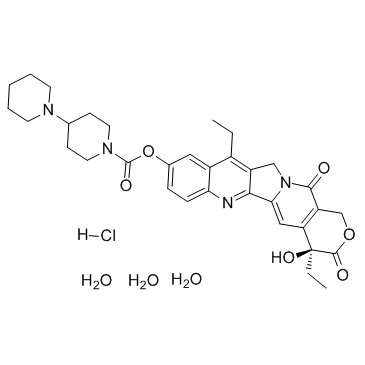 CAS#:136572-09-3
CAS#:136572-09-3