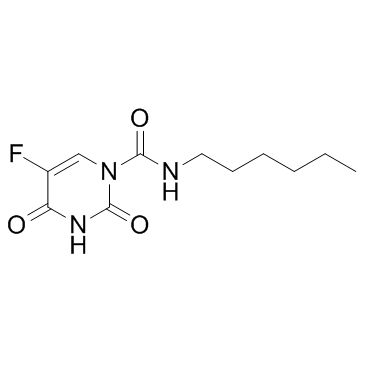
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UV7714000
-
CHEMICAL NAME :
-
1(2H)-Pyrimidinecarboxamide, 3,4-dihydro-2,4-dioxo-5-fluoro-N-hexyl-
-
CAS REGISTRY NUMBER :
-
61422-45-5
-
BEILSTEIN REFERENCE NO. :
-
0888898
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
21
-
MOLECULAR FORMULA :
-
C11-H16-F-N3-O3
-
MOLECULAR WEIGHT :
-
257.30
-
WISWESSER LINE NOTATION :
-
T6NVMVJ AVM6 EF
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1152 mg/kg/14W-I
-
TOXIC EFFECTS :
-
Brain and Coverings - encephalitis Behavioral - hallucinations, distorted perceptions Behavioral - ataxia
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
1749 mg/kg/29W-I
-
TOXIC EFFECTS :
-
Behavioral - hallucinations, distorted perceptions Behavioral - toxic psychosis Behavioral - ataxia
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
268 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
93 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
260 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1129 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold Behavioral - excitement
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
96 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
532 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
65 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rabbit
-
DOSE/DURATION :
-
55 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6 gm/kg/30D-C
-
TOXIC EFFECTS :
-
Blood - changes in erythrocyte (RBC) count Related to Chronic Data - death Related to Chronic Data - changes in testicular weight
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
182 gm/kg/26W-C
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - interstitial nephritis Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Related to Chronic Data - changes in testicular weight
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
1365 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Behavioral - food intake (animal) Nutritional and Gross Metabolic - weight loss or decreased weight gain Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
1820 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Brain and Coverings - other degenerative changes Related to Chronic Data - death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
275 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
110 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
550 mg/kg
-
SEX/DURATION :
-
female 7-17 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - growth statistics (e.g.%, reduced weight gain)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3 gm/kg
-
SEX/DURATION :
-
male 4 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
Micronucleus test
MUTATION DATA
-
TEST SYSTEM :
-
Rodent - mouse
-
DOSE/DURATION :
-
400 mg/kg
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 19,363,1980
|