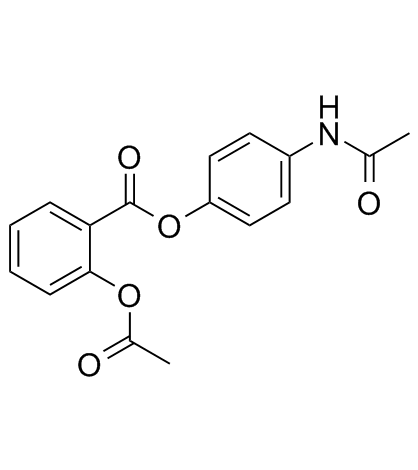
5003-48-5
| Name | 4-Acetamidophenyl 2-acetoxybenzoate |
|---|---|
| Synonyms |
Fenasprate
4'-(Acetamido)phenyl-2-acetoxybenzoate Fenasparate (1E)-N-{4-[(2-Acetoxybenzoyl)oxy]phenyl}ethanimidic acid 4-Acetamidophenyl 2-acetoxybenzoate p-Acetamidophenyl acetylsalicylate Benzoic acid, 2-(acetyloxy)-, 4-[[(1E)-1-hydroxyethylidene]amino]phenyl ester Benorilate EINECS 225-674-5 4-(acetylamino)phenyl 2-(acetyloxy)benzoate Benorylate MFCD00864257 Aspirin acetaminophen ester (4-acetamidophenyl) 2-acetyloxybenzoate Benzoic acid, 2-(acetyloxy)-, 4-(acetylamino)phenyl ester Salicylic Acid Acetate Ester with 4-Hydroxyacetanilide 4-Acetamidophenyl salicylate acetate Salipran |
| Description | Benorylate (Benoral) is the esterification product of paracetamol and acetylsalicylic acid. It has anti-inflammatory, analgesic and antipyretic properties. Benorylate could also inhibit prostaglandin (PG) synthesis. |
|---|---|
| Related Catalog | |
| Target |
Prostaglandin[4]. |
| In Vitro | Benorilate is an esterified aspirin preparation whose antirheumatic properties are reported to be as good as those of aspirin[1]. Benorylate causes a large decrease in the liver’s conversion rate of lactate into glucose, an important component of glucose homeostasis. Benorylate also impairs the urea synthesis rate from ammonia, another important function of the liver[2]. |
| In Vivo | Benorylate is probably absorbed as the intact molecule which accounts for its good gastric tolerance[3]. Benorylate could inhibit PG synthesis in laboratory animals and in human tissue[4]. |
| References |
[1]. Croft DN, et al. Gastric bleeding and benorylate, a new aspirin. Br Med J. 1972 Sep 2;3(5826):545-7. |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 511.5±60.0 °C at 760 mmHg |
| Melting Point | 177-181ºC |
| Molecular Formula | C17H15NO5 |
| Molecular Weight | 313.305 |
| Flash Point | 263.1±32.9 °C |
| Exact Mass | 313.095032 |
| PSA | 81.70000 |
| LogP | 2.22 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.566 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|---|
| HS Code | 2924299090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |