67-30-1
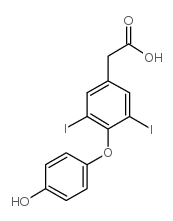
| Name | 2-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]acetic acid |
|---|---|
| Synonyms |
5-diiodophenoxy)-3
Thyroacetic acid,3,3',5,5'-tetraiodo 3,5-Diiodo-4-(4-hydroxy-3,5-diiodophenoxy)benzeneacetic acid Tetrac MFCD00055932 [4-(4-hydroxy-3 4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenylacetic acid Benzeneacetic acid,3,5-diiodo-4-(4-hydroxy-3,5-diiodophenoxy) 3,3',5,5'-TETRAIODOTHYROACETIC ACID Tetraiodothyroacetic acid Acide 3,5,3',5'-tetraiodothyroacetique [French] 5-diiodophenyl] acetic acid EINECS 200-649-1 |
| Description | Tetrac (Tetraiodothyroacetic acid), a deaminated analogue of L-thyroxine (T4), and is a thyrointegrin receptor antagonist. Tetrac blocks the actions of T3 and T4 as well as different growth factors-mediated angiogenesis. Tetra also has anticancer activity[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Tetrac (0.01-1 μM; 2-6 d) induces anti-proliferation in HT-29 and HCT116 cells with different K-RAS status[3]. Tetrac (0.1 μM; 30 min) inhibits activation of ERK1/2 in HT-29 and HCT116 cells[3]. Tetrac (0.1 μM; 24 h) inhibits expression of CCND1 and c-Myc, but promotes expression of CASP2 and THBS1[3]. Cell Proliferation Assay[3] Cell Line: HT-29 and HCT116 cells Concentration: 0.01, 0.1, 1 μM Incubation Time: 0, 2, 4, 6 days Result: Induced anti-proliferation of K-RAS wild-type colorectal cancer cells. Western Blot Analysis[3] Cell Line: HT-29 and HCT116 cells Concentration: 0.1 μM Incubation Time: 30 min Result: Inhibited constitutively activated ERK1/2, and this inhibition can remove by anti-integrin αvβ3 antibody pretreatment. |
| In Vivo | Tetrac (35 μg; p.o. for 40 days) inhibits tumor inoculation, growth and integrin expression in mice[4]. Animal Model: Wild-type male Balb/C mice aged 8 weeks are inoculated with 102B16F10 or B16LS9 cells[4] Dosage: 35 μg per day Administration: P.o. (added to the drinking water) daily for 40 days Result: Delayed the onset of ocular melanoma. Reduced the S-100 and integrin staining level in the B16F10 mice model. |
| References |
| Density | 2.727g/cm3 |
|---|---|
| Boiling Point | 544.8ºC at 760 mmHg |
| Melting Point | 230 °C |
| Molecular Formula | C14H8I4O4 |
| Molecular Weight | 747.82900 |
| Flash Point | 283.3ºC |
| Exact Mass | 747.66000 |
| PSA | 66.76000 |
| LogP | 5.23000 |
| Index of Refraction | 1.801 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+ |
| Risk Phrases | 28 |
| Safety Phrases | 45 |
| RIDADR | UN 2811 6.1/PG 1 |
| RTECS | CY1585800 |
| Hazard Class | 6.1 |
| HS Code | 2918990090 |
|
~90% 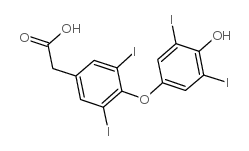
67-30-1 |
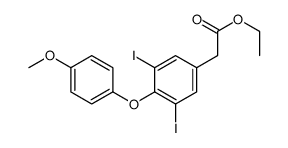
| Literature: Bridoux, Alexandre; Khan, Riaz A.; Chen, Celei; Cheve, Gwenael; Cui, Huadong; Dyskin, Evgeny; Yasri, Aziz; Mousa, Shaker A. Journal of Enzyme Inhibition and Medicinal Chemistry, 2011 , vol. 26, # 6 p. 871 - 882 |
|
~% 
67-30-1 |
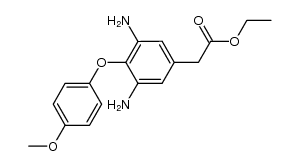
| Literature: US2011/105482 A1, ; |
|
~% 
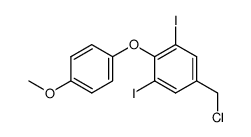
67-30-1 |
| Literature: US2011/105482 A1, ; |
|
~% 
67-30-1 |
| Literature: US2011/105482 A1, ; |
|
~% 
67-30-1 |
| Literature: Biochemical Journal, , vol. 63, p. 601,603 |
|
~% 
67-30-1 |
| Literature: US2011/105482 A1, ; |
|
~% 
67-30-1 |
| Literature: Biochemical Journal, , vol. 50, p. 438 |
|
~% 
67-30-1 |
| Literature: Biochemical Journal, , vol. 50, p. 438 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |