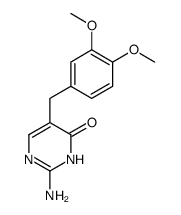
5355-16-8
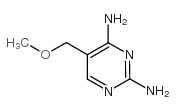
| Name | diaveridine |
|---|---|
| Synonyms |
Diaveridinum [INN-Latin]
Diaveridinum DIAVERIDINE 2,4-Diamino-5-veratrylpyrimidine 5-(3,4-Dimethoxybenzyl)-2,4-pyrimidinediamine 2,4-diamino-5-(3,4-dimethoxy-benzyl)-pyrimidine MFCD00057349 5-(3,4-dimethoxy-benzyl)-pyrimidine-2,4-diamine 5-[(3,4-dimethoxyphenyl)methyl]pyrimidine-2,4-diamine Diaveridin BW 49-210 Diaveridina 5-Veratryl-pyrimidin-2,4-diyldiamin EINECS 226-333-3 |
| Description | Diaveridine (EGIS-5645) is a dihydrofolate reductase (DHFR) inhibitor with a Ki of 11.5 nM for the wild type DHFR and also an antibacterial agent. |
|---|---|
| Related Catalog | |
| Target |
Ki: 11.5 nM (DHFR) [1] Bacterial[2] |
| In Vitro | Diaveridine is a dihydrofolate reductase (DHFR) inhibitor with a Ki of 11.5 nM for the wild type DHFR and also an antibacterial agent[1]. Treatments with Diaveridine for 90 min have a strong bactericidal effect on S. typhimurium TA1535, and no bacterial growth is observed at 10μg/mL or more. Without metabolic activation, treatment with Diaveridine for 48 h, but not 24 h, causes a dose-dependent, significant increase in the frequency of aberrant metaphases. At 100 μg/mL, 60% of the metaphases contain chromosome aberrations[2]. |
| In Vivo | The sperm abnormality of the Diaveridine (DVD) treatment groups at all dose levels (Diaveridine, 128 to 512 mg/kg) shows no significant differences compare with the negative control group. There are no significant differences of micronucleus between the negative control group and the Diaveridine treatment groups (Diaveridine, 128 to 512 mg/kg). The chromosome aberration of the Diaveridine treatment groups at all dose levels and the negative control group are significantly lower than those in the positive control group treated with cyclophosphamide (P<0.05), indicating that Diaveridine at the doses studied does not cause abnormal chromosome aberration. The results demonstrate that the Diaveridine administration does not produce significant changes in the ratio of organ-to-body weight, compare with the negative control group in the end period of the study[3]. |
| Cell Assay | Cells are cultured at 37°C in a humidified atmosphere of 5% CO2 in air. The growth medium is Eagle’s MEM supplemented with 10% fetal bovine serum. In the experiment without metabolic activation, the cells are treated for 24 or 48 h continuously without a medium change. In the experiment with metabolic activation, the cells are pulse treated with test compounds (including Diaveridine) at varying doses for 6 h and incubated for 18 h in fresh culture medium. Breakage type chromatid aberrations, exchange type chromatid aberrations, breakage type chromosome aberrations, and exchange type chromosome aberrations are scored. Gaps are also counted. Mitotic index is determined from scoring 2000 cells[2]. |
| Animal Admin | 3]Fifty male ICR mice, weighing 25 to 35 g, are assigned to five groups randomly with 10 mice in each group. Mice in the experiment groups receive Diaveridine (DVD) via IG at ed 128 mg/kg (low doses), 256 mg/kg (medium doses), and 512 mg/kg (high doses) body weight for 5 consecutive days, respectively. Mice in negative and positive control groups receive IG 1% CMC-Na solvent and 40 mg/kg body weight of cyclophosphamide, respectively. The testing groups are administered 0.2 mL/10 g Diaveridine (mixed with 1% of CMC-Na, to obtain the concentration of 2 mg/mL.) body weight, once a day, for 5 days. The behavioral changes are recorded on the daily basis[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 506.1±60.0 °C at 760 mmHg |
| Melting Point | 232 - 236ºC |
| Molecular Formula | C13H16N4O2 |
| Molecular Weight | 260.292 |
| Flash Point | 259.9±32.9 °C |
| Exact Mass | 260.127319 |
| PSA | 96.28000 |
| LogP | 1.09 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.626 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | UV8142000 |
| HS Code | 2933599090 |
|
~91% 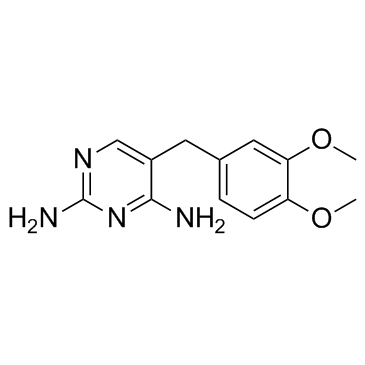
5355-16-8 |
| Literature: Hoffmann-La Roche Inc. Patent: US4143227 A1, 1979 ; |
|
~96% 
5355-16-8 |
| Literature: Hachtel; Haller; Seydel Arzneimittel-Forschung/Drug Research, 1988 , vol. 38, # 12 p. 1778 - 1783 |
|
~% 
5355-16-8 |
| Literature: Journal of the American Chemical Society, , vol. 73, p. 3758,3760 US2624732 , ; |
|
~% 
5355-16-8 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 38, # 12 p. 1778 - 1783 |
|
~% 
5355-16-8 |
| Literature: Arzneimittel-Forschung/Drug Research, , vol. 38, # 12 p. 1778 - 1783 |
| Precursor 7 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |