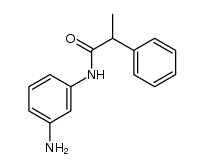
1105698-15-4
| Name | N-[3-[(2-oxonaphthalen-1-ylidene)methylamino]phenyl]-2-phenylpropanamide |
|---|---|
| Synonyms |
N-(3-{(E)-[(2-Hydroxy-1-naphthyl)methylene]amino}phenyl)-2-phenylpropanamide
Salermide Benzeneacetamide, N-[3-[[(1E)-(2-hydroxy-1-naphthalenyl)methylene]amino]phenyl]-α-methyl- |
| Description | Salermide is an inhibitor of Sirt1 and Sirt2; can cause strong cancer-specific apoptotic cell death. |
|---|---|
| Related Catalog | |
| Target |
SIRT1 SIRT2 |
| In Vitro | Salermide shows a dose-dependent inhibition that rises to 80% at 90 μM and 25 μM against Sirt1 and Sirt2, respectively. Salermide can prompt tumour-specific cell death in a wide range of human cancer cell lines derived from leukaemia (MOLT4, KG1A, K562), lymphoma (Raji), colon (SW480) and breast (MDA-MB-231). Incubation with 100 μM Salermide alone resulted in an increase of cytosolicactivated caspase 3 and a decrease of mitochondrialcytochrome. Salermide alone can induce apoptosis through both extrinsic and intrinsic pathways. Salermide had several antitumorigenic advantages over the earlier described class III HDAC inhibitors: firstly, it mimics the universal proapoptotic effect on cancer samples exhibited by the classical class I, II and IV HDAC inhibitors, and secondly, its proapoptotic effect is cancer-specific[1]. |
| In Vivo | Salermide is well tolerated by mice at concentrations up to 100 μM. Salermide's mechanism of action in vivo is specifically mediated by Sirt1. Intraperitoneal feeding of Salermide has no apparent toxicity in nude mice[1]. |
| Cell Assay | Cell lines (SW480, MDA-MB-231, MOLT4, KG1A, K562 and Raji) are used in the study. Cell viability is determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. IC50 index is calculated using four Salermide concentrations (25, 50, 75 and 100 μM) for 24 h. The percentage of apoptotic cells is determined with the FACSCalibur apparatus[1]. |
| Animal Admin | Mice: To assess possible adverse effects of Salermide in vivo. To do this, a group of 10 nude mice are intraperitoneal injected 100 μL of 100 μM of Salermide to over 34 days. Diet consumption, body-weight gain, and postural and behavioural changes are monitored throughout the study[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 666.7±50.0 °C at 760 mmHg |
| Molecular Formula | C26H22N2O2 |
| Molecular Weight | 394.465 |
| Flash Point | 357.0±30.1 °C |
| Exact Mass | 394.168121 |
| PSA | 61.69000 |
| LogP | 5.40 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.623 |
| Storage condition | -20℃ |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H317-H412 |
| Precautionary Statements | P273-P280 |
| RIDADR | NONH for all modes of transport |
|
~86% 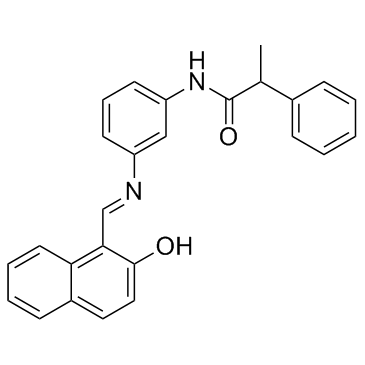
1105698-15-4 |
| Literature: Rotili, Dante; Tarantino, Domenico; Nebbioso, Angela; Paolini, Chantal; Huidobro, Covadonga; Lara, Ester; Mellini, Paolo; Lenoci, Alessia; Pezzi, Riccardo; Botta, Giorgia; Lahtela-Kakkonen, Maija; Poso, Antti; Steinkuehler, Christian; Gallinari, Paola; De Maria, Ruggero; Fraga, Mario; Esteller, Manel; Altucci, Lucia; Mai, Antonello Journal of Medicinal Chemistry, 2012 , vol. 55, # 24 p. 10937 - 10947 |
|
~% 
1105698-15-4 |
| Literature: Rotili, Dante; Tarantino, Domenico; Nebbioso, Angela; Paolini, Chantal; Huidobro, Covadonga; Lara, Ester; Mellini, Paolo; Lenoci, Alessia; Pezzi, Riccardo; Botta, Giorgia; Lahtela-Kakkonen, Maija; Poso, Antti; Steinkuehler, Christian; Gallinari, Paola; De Maria, Ruggero; Fraga, Mario; Esteller, Manel; Altucci, Lucia; Mai, Antonello Journal of Medicinal Chemistry, 2012 , vol. 55, # 24 p. 10937 - 10947 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |