1-甲基-1H-吡唑-5-羧酸
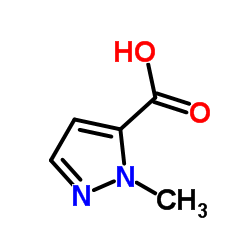
1-甲基-1H-吡唑-5-羧酸结构式

|
常用名 | 1-甲基-1H-吡唑-5-羧酸 | 英文名 | 1-Methylpyrazole-5-carboxylic Acid |
|---|---|---|---|---|
| CAS号 | 16034-46-1 | 分子量 | 126.11 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 306.9±15.0 °C at 760 mmHg | |
| 分子式 | C5H6N2O2 | 熔点 | 223.5-224.5 | |
| MSDS | 中文版 美版 | 闪点 | 139.4±20.4 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
1-甲基-1H-吡唑-5-羧酸用途1-甲基-5-吡唑羧酸是一种生物化学试剂,可作为生物材料或有机化合物用于生命科学相关研究。 |
| 中文名 | 1-甲基吡唑-5-甲酸 |
|---|---|
| 英文名 | 1-Methylpyrazole-5-carboxylic Acid |
| 中文别名 | 1-甲基吡唑-5-羧酸 | 1-甲基-吡唑-5-羧酸 | 1-甲基-1H-吡唑-5-羧酸 |
| 英文别名 | 更多 |
| 密度 | 1.3±0.1 g/cm3 |
|---|---|
| 沸点 | 306.9±15.0 °C at 760 mmHg |
| 熔点 | 223.5-224.5 |
| 分子式 | C5H6N2O2 |
| 分子量 | 126.11 |
| 闪点 | 139.4±20.4 °C |
| 精确质量 | 126.042931 |
| PSA | 55.12000 |
| LogP | -0.36 |
| 外观性状 | 固体;White to Almost white powder to crystal |
| 蒸汽压 | 0.0±0.7 mmHg at 25°C |
| 折射率 | 1.591 |
| 储存条件 | 室温,干燥,密封 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):0.1 2.氢键供体数量:1 3.氢键受体数量:3 4.可旋转化学键数量:1 5.互变异构体数量:无 6.拓扑分子极性表面积55.1 7.重原子数量:9 8.表面电荷:0 9.复杂度:126 10.同位素原子数量:0 11.确定原子立构中心数量:0 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1.熔点(ºC):220~225 |
| 符号 |

GHS07 |
|---|---|
| 信号词 | Warning |
| 危害声明 | H315-H319-H335 |
| 警示性声明 | P261-P305 + P351 + P338 |
| 危害码 (欧洲) | Xi: Irritant; |
| 风险声明 (欧洲) | R36/37/38 |
| 安全声明 (欧洲) | 37/39-26 |
| 危险品运输编码 | NONH for all modes of transport |
| 海关编码 | 2933199090 |
|
~44% 
1-甲基-1H-吡唑-5-羧酸 16034-46-1 |
| 文献:Galapagos NV Patent: WO2009/71706 A1, 2009 ; Location in patent: Page/Page column 48 ; WO 2009/071706 A1 |
|
~38% 
1-甲基-1H-吡唑-5-羧酸 16034-46-1 |
| 文献:J. Gen. Chem. USSR (Engl. Transl.), , vol. 52, # 11 p. 2592 - 2598,2291 - 2296 |
|
~27% 
1-甲基-1H-吡唑-5-羧酸 16034-46-1 |
| 文献:Takeda Pharmaceutical Company Limited Patent: EP2530078 A1, 2012 ; Location in patent: Page/Page column 64 ; |
|
~77% 
1-甲基-1H-吡唑-5-羧酸 16034-46-1 |
| 文献:Manaev, Yu. A.; Andreeva, M. A.; Perevalov, V. P.; Stepanov, B. I.; Dubrovskaya, V. A.; Seraya, V. I. J. Gen. Chem. USSR (Engl. Transl.), 1982 , vol. 52, # 11 p. 2592 - 2598,2291 - 2296 |
|
~% 
1-甲基-1H-吡唑-5-羧酸 16034-46-1 |
| 文献:Journal of the American Chemical Society, , vol. 80, p. 6271,6273 Justus Liebigs Annalen der Chemie, , vol. 625, p. 55,60 |
|
~99% 
1-甲基-1H-吡唑-5-羧酸 16034-46-1 |
| 文献:Lyalin; Petrosyan Russian Chemical Bulletin, 2012 , vol. 61, # 6 p. 1148 - 1153 Izv. Akad. Nauk, Ser. Khim., 2012 , vol. 61, # 6 p. 1139 - 1143,5 |
|
~% 
1-甲基-1H-吡唑-5-羧酸 16034-46-1 |
| 文献:Journal of the American Chemical Society, , vol. 80, p. 6271,6273 Justus Liebigs Annalen der Chemie, , vol. 625, p. 55,60 |
|
~% 
1-甲基-1H-吡唑-5-羧酸 16034-46-1 |
| 文献:Journal of the American Chemical Society, , vol. 80, p. 6271,6273 Justus Liebigs Annalen der Chemie, , vol. 625, p. 55,60 |
| 1-甲基-1H-吡唑-5-羧酸上游产品 10 | |
|---|---|
| 1-甲基-1H-吡唑-5-羧酸下游产品 10 | |
| 海关编码 | 2933199090 |
|---|---|
| 中文概述 | 2933199090. 其他结构上有非稠合吡唑环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途, 乌洛托品请注明外观, 6-己内酰胺请注明外观, 签约日期 |
| Summary | 2933199090. other compounds containing an unfused pyrazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
The aryl hydrocarbon receptor suppresses osteoblast proliferation and differentiation through the activation of the ERK signaling pathway.
Toxicol. Appl. Pharmacol. 280(3) , 502-10, (2014) Ahr activation is known to be associated with synovitis and exacerbated rheumatoid arthritis (RA), but its contributions to bone loss have not been completely elucidated. Osteoblast proliferation and ... |
|
|
Mono-substituted isopropylated triaryl phosphate, a major component of Firemaster 550, is an AHR agonist that exhibits AHR-independent cardiotoxicity in zebrafish.
Aquat. Toxicol. 154 , 71-9, (2014) Firemaster 550 (FM550) is an additive flame retardant mixture used within polyurethane foam and is increasingly found in house dust and the environment due to leaching. Despite the widespread use of F... |
| 2-methylpyrazole-3-carboxylic acid |
| 1-Methyl-pyrazole-5-carboxylicacid |
| N-Methylpyrazole-3-carboxylic acid |
| 1H-Pyrazole-5-carboxylic acid, 1-methyl- |
| N-methyl-pyrazole-3-carboxylic acid |
| MFCD00464253 |
| 1-Methylpyrazole-5-carboxylic acid |
| 2H-Pyrazole-3-carboxylic acid, 2-methyl- |
| 1-Methyl-1H-pyrazole-5-carboxylic acid |
| 2-Methyl-2H-pyrazole-3-carboxylic acid |
| T5NNJ A1 EVQ |







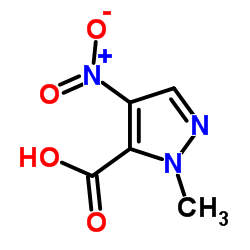 CAS号92534-69-5
CAS号92534-69-5 CAS号92534-73-1
CAS号92534-73-1 CAS号84547-61-5
CAS号84547-61-5 CAS号84547-84-2
CAS号84547-84-2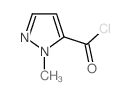 CAS号84547-59-1
CAS号84547-59-1 CAS号84547-83-1
CAS号84547-83-1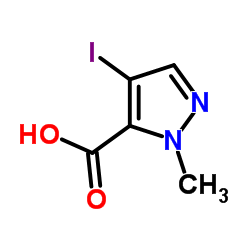 CAS号75092-30-7
CAS号75092-30-7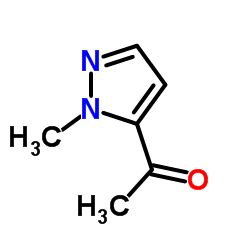 CAS号137890-05-2
CAS号137890-05-2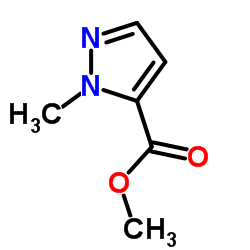 CAS号17827-60-0
CAS号17827-60-0
